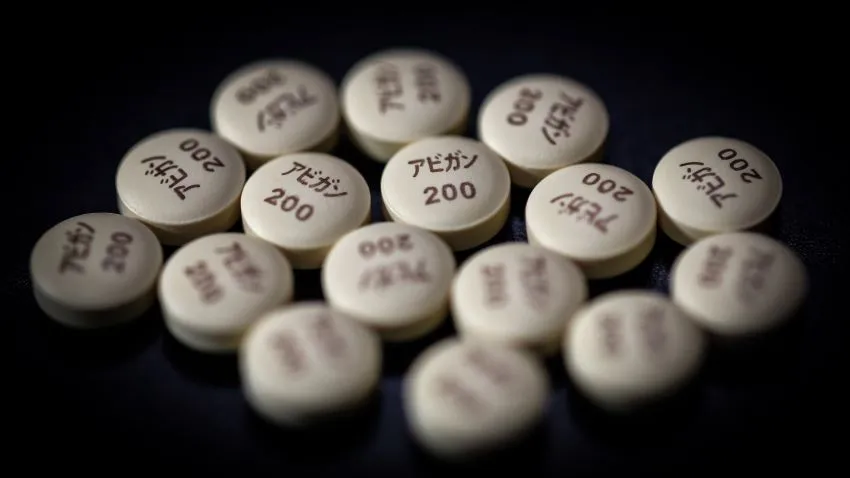
Fujifilm Holdings on Monday said it was preparing to increase production of its flu drug Avigan, after Japanese Prime Minister Shinzo Abe expressed support for using the medication as a treatment for the new coronavirus.
Clinical trials begin Tuesday. Some doctors in Japan already administer Avigan for research purposes, but company-led clinical trials are a must to gain approval for wide distribution as a treatment.
The trials will be conducted by subsidiary Fujifilm Toyama Chemical on around 100 people.
The Japanese government has a strategic reserve of 2 million doses of Avigan, but this might not be enough for all coronavirus patients.
Fujifilm restarted production of Avigan in early March. To raise output, the company is asking for help from raw material suppliers and is considering outsourcing part of the manufacturing process.
Shares of Fujifilm Holdings jumped on the news, rising 11% to 5,549 at one point on Monday in Tokyo.
Avigan, developed by Toyama Chemical, gained attention earlier in March, after a Chinese official said clinical trials indicated the drug is effective in treating the coronavirus.
Abe suggested on Saturday that Japan would follow China's lead in considering Avigan as a coronavirus treatment, saying Japan "will expand clinical research" on Avigan and "start increasing drug production."
"We also plan to launch the clinical trial process required for formal approval [of treatments for] novel-coronavirus infection," Abe added.
Abe spoke with World Health Organization Director-General Tedros Adhanom Ghebreyesus by phone Monday, where the Japanese leader explained that the country will be conducting clinical trials for Avigan for it to be officially approved as a coronavirus drug.
Tedros said that the WHO is determined to coordinate among countries to develop various drugs and vaccines for the coronavirus.
Avigan was approved by Japanese regulators in 2014. But unlike other flu drugs such as Tamiflu, it has not been in continuous production because it is only used to treat new or reemerging influenza viruses, and at the request of the health minister.
Earlier in March the Chinese government said Avigan proved effective against the virus in clinical trials. No obvious side effects were reported. However, some researchers have warned Avigan needs to be used with great care because it can cause serious fetal abnormalities if taken by pregnant women.