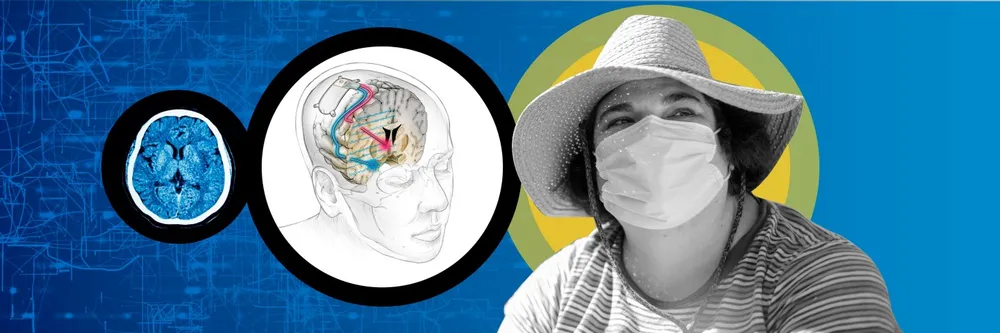
Sarah had endured five years of torture from severe and untreatable depression. “Each day I forced myself to resist the suicidal impulses that overtook me several times an hour,” the 36-year-old Californian says.
Then a surgical team at the University of California, San Francisco, inserted a thin wire deep into her brain — and administered a mild electric pulse. “When I first received the stimulation, the ‘aha’ moment occurred,” says Sarah, who wants only to be known by her first name. “I felt the most intensely joyous sensation and my depression was a distant nightmare for a moment.”
After the UCSF scientists had discovered which part of Sarah’s brain was associated with negative feelings and which would respond to a relieving stimulus, they incorporated their findings in a permanent implant that acts like a neural pacemaker. Having worn the device for a year, she reports that it has “kept depression at bay, allowing me to return to my best self and rebuild a life worth living”.
The UCSF researchers hailed their achievement, published on Monday in Nature Medicine, as a landmark in the effort to develop personalised treatments for depression through neural electronics. For the first time researchers have identified and modulated a brain circuit uniquely associated with the symptoms. Until now, procedures that apply electricity to the patient’s brain have taken a “one-size-fits-all” approach.

Neuroscientists who were not involved in the project agreed that it was a significant step forward, while cautioning that many years of work would be needed to convert an expensive and time-consuming surgical procedure into something that could be applied more widely to intractable depression. Patients with other psychiatric conditions may benefit too from personalised deep brain stimulation, as well as those with Parkinson’s disease and epilepsy who are already treated with DBS.
Sameer Sheth, a neurosurgeon at Baylor College of Medicine in Texas, says he is “super-excited” by the UCSF work — adding that he is carrying out a similar neurotechnology trial in intractable depression, which has given equally encouraging results with the first participant, though they have not yet been published. “We all recognise that some patients need an individualised approach,” he says.
Finding the ‘biomarker’
The unmet medical need is huge. According to the World Health Organization, 280m people globally suffer from serious depression, of whom about 30 per cent do not respond well to existing treatments: psychotherapy, antidepressant drugs or electroconvulsive therapy. And evidence is emerging that the Covid-19 pandemic has substantially increased the incidence of depression and other mental health problems for all age groups, including children.
Electroconvulsive therapy was the first psychiatric treatment using electricity, though it works in a very different way to the individualised deep brain stimulation used in the UCSF and Baylor trials.
ECT still suffers from a “brain frying” reputation from the mid-20th century, when large pulses were given without anaesthesia, sometimes leading to memory loss, fractured bones and other serious side effects. Today ECT is carried out under general anaesthesia, with milder currents passed through the brain from electrodes on the scalp, triggering a brief seizure that somehow resets neural circuitry or chemistry. No one really knows how it works — and it does not benefit everyone — but an estimated 100,000 people a year undergo ECT in the US alone.

The UCSF and Baylor teams are not the first to study deep brain stimulation for depression, but previous trials with simpler devices that delivered a continuous stimulus in one place have given mixed results. Two DBS trials sponsored by medical technology companies were stopped early because they were not delivering clear benefits.
“It might have been naive of us to think that such a heterogeneous condition as depression could be treated in the same way for different patients,” says Darin Dougherty, director of neurotherapeutics at Massachusetts General Hospital, who led one of the discontinued trials.
DBS has become a routine and effective treatment for epilepsy and Parkinson’s disease, in which scientists know which brain regions to target. The problem with applying neuro-electronics to depression in the same way has been that scientists do not know enough about the particular brain circuits associated with the condition.
The UCSF team’s key discovery was a “biomarker” indicating the onset of depressive symptoms, a specific pattern of neural activity in a brain area called the amygdala that deals with responses to threats. A stimulus there did not relieve Sarah’s symptoms, but the researchers found another place, the ventral striatum, where tiny electric pulses immediately lifted unwanted feelings when they were detected in the amygdala. Controlling the electronics is a matchbox-sized device adapted from a $30,000 implant commercially available for epilepsy.
“This is an exciting step forward due to the bespoke nature of the stimulation,” says Jonathan Roiser, professor of neuroscience and mental health at University College London. “It is likely that if trialled in other patients, different recording and stimulation sites would be required, as the precise brain circuitry underlying symptoms probably varies between individuals.”
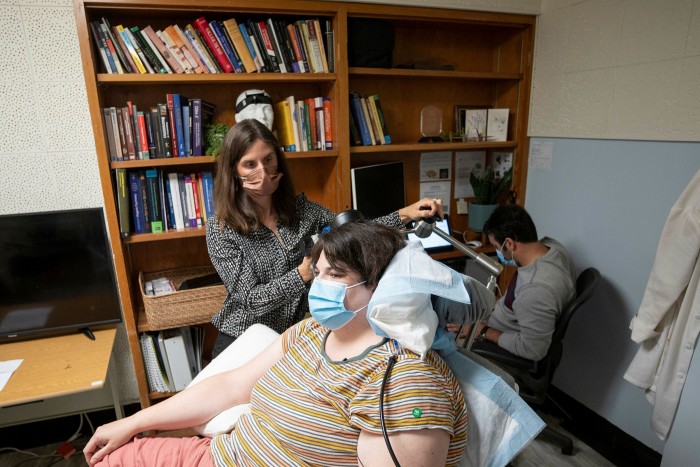
Katherine Scangos, the psychiatrist leading the UCSF trial, has recruited two more patients with severe depression to take part and aims for 12 volunteers altogether. “We have a lot left to learn about variability across different patients and different types of depression,” she says.
Even if the clinical trials of personalised DBS for depression under way at UCSF and Baylor show that the technology works as well in other cases as for Sarah, “this kind of highly invasive surgical procedure would only ever be used in the most severe patients with intractable symptoms”, says Roiser.
But UCSF neurosurgeon Ed Chang points out that there is great scope for improving DBS technology, which is based on the electronic circuitry developed for cardiac pacemakers 30 to 40 years ago. “What we are doing now is relatively crude compared to what I think the future can offer,” he says.
UCSF scientists are also thinking about patent rights. “Companies that make medical devices will need some intellectual property or patents in order to invest the resources — tens of millions of dollars — to do a formal trial,” Chang says. “We are looking into that because we are very serious about trying to understand if this could be a future therapy.”
Looking further ahead, researchers hope that insights into the brain circuits involved in depression gained from personalised DBS could be used to develop non-invasive electronic treatments. “We really hope this is going to be something that can scale to other technologies in the future that may not even exist now,” Chang says.
Electrodes in the UCSF trial stimulate brain regions just a millimetre or 1.5mm across. There is no obvious way to achieve such precision with non-invasive targeting from outside the skull, though highly focused ultrasound beams are one possibility.
Roiser points out that two existing non-invasive electronic treatments, both milder in their impact than ECT, do improve symptoms in many patients though neither has a reliable effect on severe, intractable depression.
The stronger of the two is transcranial magnetic stimulation (TMS), in which a coil placed against the scalp delivers a magnetic pulse to stimulate nerve cells in the brain region involved in mood control. The gentlest method of stimulating the brain electrically is transcranial direct current stimulation (tDCS), which sends a weak electric current across the brain.
“There have been some studies trying to make these non-invasive techniques into better and more bespoke treatments,” Roiser says. But they do not come close to the new DBS methods in their precision.

‘Virtuous spiral upwards’
Another form of neurotechnology that is not yet applied to psychiatry but might be in the future is the brain-computer interface (BCI), which requires more data processing power than the electronic devices used to treat depression.
This field of research has concentrated so far on recording neural activity for one-way communication from the brain to an outside device. For example if the BCI picks up an intention to move a limb, severely handicapped patients can drive a robotic arm or artificially stimulate their own muscles to bypass a spinal cord injury. The technology also promises to open a window into the mind of people whose brain is too damaged to communicate with the outside world in any other way.
Two-way communications between brain and computer could eventually help people with psychiatric and neurological disorders. The best known and most richly funded BCI business, Elon Musk’s Neuralink, aims to move on from helping quadriplegics communicate to “treat various brain-related ailments”, the company said as it raised another $205m in a series C funding round in August.
But all that lies well into the future. “We should stress that we are at a very early stage in developing BCIs,” says Roiser, “and the idea that we’ll eventually be able to read the human neural code is very far-fetched.”
For now Sarah is thrilled with the new life that her DBS device has given her. “Depression controlled my life. I barely moved and barely did anything. I had to . . . relearn activities and the things I love to do,” she says. “It has been a virtuous spiral upwards. Everything has gotten easier and easier and easier.”
Sarah also hopes the project will help to overcome the widespread stigma attached to people with depression, “the feedback we get from those around us in society that it’s a moral failing”.
Scangos, her psychiatrist, says the inscrutable causes of depression do not help either. “I think some of the stigma comes from the ‘black box’ nature of the disorder,” she says. “We hope this will resolve as we begin to define the biological substrates of depression and hopefully this study will allow us to do so one patient at a time.”