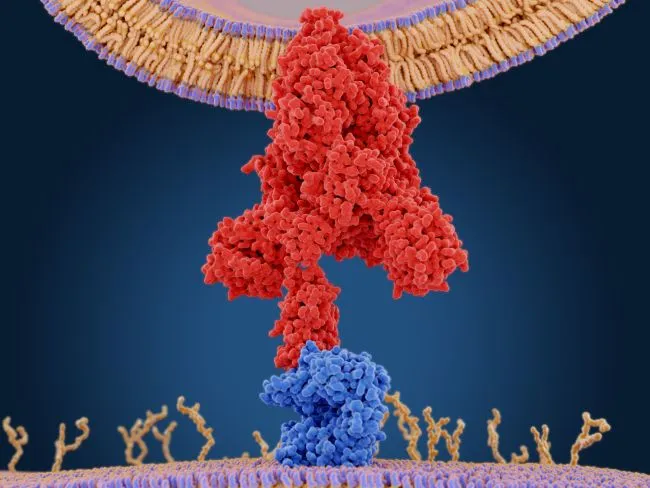
The protein that the coronavirus uses to attach to human cells has a compact "ridge" that allows it to attach more strongly to human cells than similar viruses, allowing it to infect better and spread faster, according to a new study.
The new coronavirus, SARS-CoV-2, attaches to human cells through what's called a "spike protein," according to a previous Live Science report. After the spike protein binds to the human cell receptor — a protein on the cell surface that serves as a door into the cell — the viral membrane fuses with the human cell membrane, allowing the genome of the virus to enter human cells.
All coronaviruses attach to human cells through spike proteins, but different coronaviruses have spike proteins with different structures. In February, a group of researchers at the University of Texas at Austin and at the National Institutes of Health mapped out the molecular structure of the new coronavirus' spike protein, according to the report.
Now, another group of researchers used X-rays to further explore the coronavirus' spike protein and the human cell receptor it binds to. Their goal was to understand why this coronavirus' spike protein is so good at infecting cells, compared with a similar coronavirus, known as SARS-CoV, that caused the severe acute respiratory syndrome (SARS) outbreak in 2003.
This more compact structure, and several other small differences, allows SARS-CoV-2 to attach more strongly to the human ACE2 receptor, thereby enabling it to better infect cells and thus spread faster than the SARS coronavirus, according to the statement.
"In general, by learning what structural features of viral proteins are most important in establishing contact with human cells, we can design drugs that seek them out and block their activity — like jamming their radar," Fang Li, a professor in the Department of Veterinary and Biomedical Sciences at the University of Minnesota, said in the statement.
By studying the specifics of this virus and how it attaches to cells, the researchers also gained some insights into how the virus may have hopped from animals to humans.
They discovered that a bat coronavirus also binds to the ACE2 receptor, but poorly. A few mutations could have increased the ability of the bat virus to attach to the human receptor, allowing the hop to humans, according to the statement. The researchers also analyzed the structure of the spike proteins of pangolins, which could be an intermediate host between bats and humans, according to a previous Live Science report.
They found that one of the pangolin coronaviruses could potentially bind to the human receptor, supporting the notion that pangolins were intermediate hosts of the virus. But that hypothesis would "need to be verified experimentally," they wrote in the study.
The findings were published March 30 in the journal Nature.