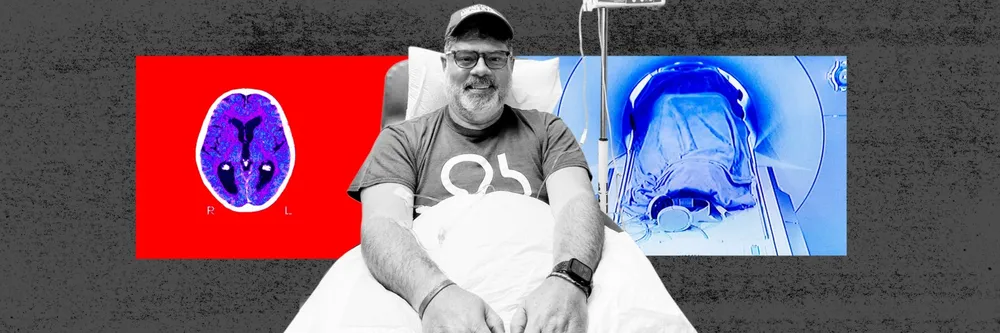
Jeff Borghoff knew something was wrong after his face developed a twitch, then a droop. When at the age of just 51 he received the devastating diagnosis of early onset Alzheimer’s disease, his biggest dread was a rapid deterioration in his mental powers.
Desperate to keep the condition at bay, the former IT executive signed up for the clinical trial of Biogen’s experimental Alzheimer’s drug, aducanumab. Six years on, Borghoff credits the controversial treatment for valuable extra time with his wife and his three children.
“Our fear all along was that there was going to be a steep decline in all of my mental faculties, but to date that has not been the case,” he says. “I’ve had some symptomatic issues . . . but the medication really is about more time, more time with my loved ones, those are critical things.”
Now, following the drug’s approval this week by the US medicines regulator, many of the estimated 35m living with Alzheimer’s around the world will be waiting in line for their own infusion of hope.
Yet there is a sting in the tail on what might seem like unambiguously good news for patients. A series of problems, ranging from the cost of the drug to questions about the evidence of its efficacy, will pile pressure on health systems already battered by the pandemic and which may be ill-equipped to meet the expectations that have been raised.

The approval of the drug risks opening a political and business divide between the US — where a price tag of $56,000 a year has been set — and Europe, where many governments may be far more sceptical of its value.
Complicating the debate is a growing chorus of anger over the FDA’s decision to green light the drug in the first place, given the scant evidence of its efficacy. Three leading scientists resigned from its advisory board this week, in an extraordinary repudiation of a supposedly objective and data-driven approval process.
Biogen originally abandoned the drug after a futility analysis suggested it was ineffective. But when it examined a larger data set, it found patients taking a higher dose “experienced significant benefits on measures of cognition and function such as memory, orientation, and language”.
Craig Garthwaite, professor of hospital and health services at Northwestern University, says it is “terrible” that the FDA has bowed to patient pressure groups and not listened to its scientific advisers. He says he is “puzzled” by “this idea that even if we don’t know or even if it doesn’t work we should try it to give people hope”.
Yet experts believe health insurers are likely to feel obliged to cover the drug now it has been approved by the FDA. In an unexpected twist, the regulator approved it for all patients with the condition — rather than just for those with the early stage of the disease, who were studied in the clinical trial.

Most US patients will be on Medicare, the public health insurer for the over 65s, which Biogen told investors it expects to cover the “vast majority” of patients. Some experts predict it will quickly become Medicare’s largest expenditure for physician-administered drugs.
The potential costs go well beyond just the medicine. The exigencies of administering it look set to challenge established patterns of caring for those with dementia, creating a need for different kinds of staff and expensive equipment not typically associated with treating the disease.
The cost of the intravenous infusion of the medicine, radiology and imaging could add between $2,000 and $15,000 or even more on top of the drug’s pricetag, according to estimates from Premier, a group that represents more than 4,000 US hospitals.
David Thomas, head of policy for Alzheimer’s Research UK, says that “a lot of care for people with dementia [in the UK] is done from memory clinics, which are largely staffed by old-age psychiatrists”. Unlike a neurologist, these doctors “often don’t have experience of the diagnostics and monitoring required to administer disease modifying treatments”.
The other big challenge, he points out, is securing the necessary equipment. Diagnoses for those suspected of having the disease customarily involve low-key cognitive tests, requiring no special hardware.
In order to be eligible for aducanumab, a patient must have a certain level in their brain of amyloids, proteins that can build up in tissues or organs, which is established through either a positron emission tomography (PET) scan or the more invasive, but typically cheaper, lumbar tap, which involves removing fluid from the spine.
Eligible patients must then undergo intravenous infusion once every four weeks with aducanumab and get regular MRI scans which can detect dangerous side effects such as brain swelling and bleeding.
When Alzheimer’s Research UK polled psychiatrists in the country recently, “the majority said that it would take up to five years to be ready to administer a treatment — only a third thought they could do it in a year”, Thomas says.
Sanjiv Sharma, Borghoff’s doctor and founder of the Advanced Memory Institute of New Jersey, acknowledges the obstacles that lie ahead if it is to reach all those who could potentially benefit. But he is in no doubt that the US must blaze the trail. “If we can’t do it [here], as the most developed country in the world, where can we do it?”, he says.

‘A financial incentive to use the drug’
Soeren Mattke, director of the Center for Improving Chronic Illness Care at the University of Southern California, argues that few developed nations are currently well prepared to administer the drug — let alone middle-income and emerging nations such as China and Brazil with far less sophisticated health infrastructure.
However, in the US ready funding for the treatment will spawn its own business models, he suggests. Medicare pays doctors a fee of 6 per cent of the price of the drug, plus infusion and visit fees.
“The US is very entrepreneurial so once . . . the neurologists or psychiatrists or geriatricians realise, ‘well, I can actually make a good living off this just by distributing the drug via my practice’, that’s a very powerful draw to institute the diagnostic facilities to find the patients,” he says.
This may bring its own conflicts of interest, suggests Northwestern’s Garthwaite. “There’s an actual financial incentive to use the drug even if you don’t think it is going to work.”
Mattke also acknowledges that the same incentives do not exist in more regimented, publicly-funded health systems such as Canada and the UK, where lengthy approvals and limited budgets may constrain the purchase of equipment, or ability to hire new staff.
“I’m a bit nervous about government-instituted change because we all know that governments don’t act very fast,” he adds.

In the US, Biogen has helped to prepare more than 900 infusion sites across the US to deliver the drug. It anticipates “modest” revenue in 2021, but then a multibillion-dollar opportunity in years to come, as it could be given to between 1m and 2m patients in the US alone.
Roni Christopher, vice-president of design and intervention in the analytics group at Premier, has spent the past year examining everything from developing standard assessments of a patient’s cognitive state to training radiologists to spot side effects, and even assessing if infusion sites have enough seating.
Even with these logistical insights, the most experienced health systems may not be ready to treat patients until the autumn. The FDA decision to open the drug up to all Alzheimer’s patients, will lead to a “bigger flood” of interest, Christopher says.
“Consumer or patient pressure is going to dictate a lot here, because it’s such a devastating disease,” she adds.

Value for money?
Bigger, even, than the question of how the drug is to be delivered is how much health systems — and the taxpayers who fund them — will be willing to pay for a medicine with such little proven impact.
In parts of Europe, where so-called “health technology assessments” are used to assess value for money of a new medicine, the battle has yet to be seriously joined over whether the drug should be offered to patients. In the UK, whose National Institute for Health and Care Excellence carries a lot of international influence, officials are privately concerned that expectations are being raised that will not easily be met.
Umer Raffat, an analyst at Evercore ISI, expects that even if aducanumab gets the green light from European authorities, they are “not going to allow a price of more than $10,000” per year, which will put Biogen in the difficult position of deciding whether to sell to the US at five times the European price.
This difference in approach looks set to inflame Republican politicians who believe that other countries which negotiate cheaper drug prices are in effect freeloading off American innovation.
On the other side of the political aisle, some Democrats are using the price Biogen has set as evidence that Medicare should be allowed to negotiate prices — a potential bargaining power with the drugs industry that the government chooses not too exercise.
Murray Aitken, executive director of the IQVIA Institute for Human Data Science in New Jersey, believes the question of how much health systems should pay for a drug that, however flawed, offers a rare shaft of hope, may prompt a long overdue public reckoning over whether European governments should spend a higher share of gross domestic product on health.
He adds: “It may precipitate a larger conversation for us as a civil society, in terms of how we’re allocating our wealth . . . It may trigger that broader social debate and discussion, which I would say is probably overdue.”
At home in New Jersey, Jeff Borghoff has no doubt about the value of the medicine which he believes has extended his lease on a meaningful life. “I know that it’s not a cure and it probably won’t fix everything that’s been damaged in my brain from Alzheimer’s,” says Borghoff. He adds: “I’m 57. I’ll be OK if I live into my 70s, that’ll be a good run and I’m hoping the medication will help me do that.”